FDA approves Roche's Gazyva/Gazyvaro for the treatment of lupus nephritis
- Bias Rating
- Reliability
40% ReliableAverage
- Policy Leaning
-16% Somewhat Left
- Politician Portrayal
N/A
Continue For Free
Create your free account to see the in-depth bias analytics and more.
By creating an account, you agree to our Terms and Privacy Policy, and subscribe to email updates.
Bias Score Analysis
The A.I. bias rating includes policy and politician portrayal leanings based on the author’s tone found in the article using machine learning. Bias scores are on a scale of -100% to 100% with higher negative scores being more liberal and higher positive scores being more conservative, and 0% being neutral.
Sentiments
60% Positive
- Liberal
| Sentence | Sentiment | Bias |
|---|---|---|
Unlock this feature by upgrading to the Pro plan. | ||
Reliability Score Analysis
Policy Leaning Analysis
Politician Portrayal Analysis
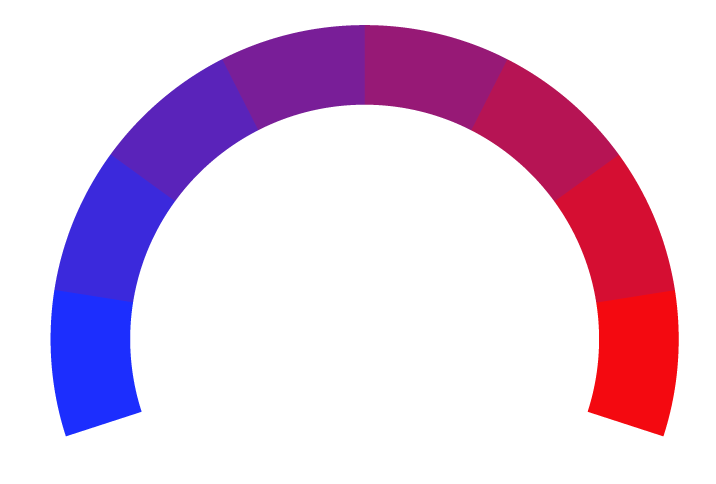
Bias Meter
Extremely
Liberal
Very
Liberal
Moderately
Liberal
Somewhat Liberal
Center
Somewhat Conservative
Moderately
Conservative
Very
Conservative
Extremely
Conservative
-100%
Liberal
100%
Conservative
Contributing sentiments towards policy:
58% : "People with lupus nephritis who achieve a complete renal response are more likely to experience preserved kidney function and delay, or even prevention, of progression to end-stage kidney disease," said Levi Garraway, MD, PhD, Roche's Chief Medical Officer and Head of Global Product Development.*Our bias meter rating uses data science including sentiment analysis, machine learning and our proprietary algorithm for determining biases in news articles. Bias scores are on a scale of -100% to 100% with higher negative scores being more liberal and higher positive scores being more conservative, and 0% being neutral. The rating is an independent analysis and is not affiliated nor sponsored by the news source or any other organization.